Healthcare organizations rely on the quality of laboratory results, and today, quality isn't only about having the latest equipment. Laboratory information management systems (LIMS) can become an essential tool to make your lab more efficient.
This article shows why creating a LIMS system is a worthwhile investment and how your medical business can profit from it.
In this article, we'll talk about managing data, automating repetitive tasks, and achieving better compliance.
Read on to find out what a LIMS is, learn about its features, and get a glimpse into the process of building such a system. We have a lot to cover, so let's dive right in.
LIMS market overview

A laboratory information management system is a multifunctional software solution that helps an organization handle lab samples and the associated data. A properly designed LIMS facilitates every aspect of the process, from the moment samples come, through data recording and storing, all the way to reporting and archiving.
Healthcare has lately been enjoying steady growth of innovations. A lot of them are centered around the industry's most valuable commodity — data. EMR/EHR (electronic medical/health records), PMS (practice management systems), and CDSS (clinical decision support systems) are only a few examples of software that gives healthcare providers better control of their data.
LIMS software deserves to be on that list as well. Any hospital, pharmaceutical research facility, or biotech startup can benefit from a system that optimizes the digital flow of laboratory data. It's especially relevant in the pandemic reality when the number of lab tests for Covid-19 and secondary infections has skyrocketed. Just to give you a sense of perspective: data compiled by Statista shows that as of January 24, 2022, a total of 880 million Covid-19 tests have been performed in the US alone.
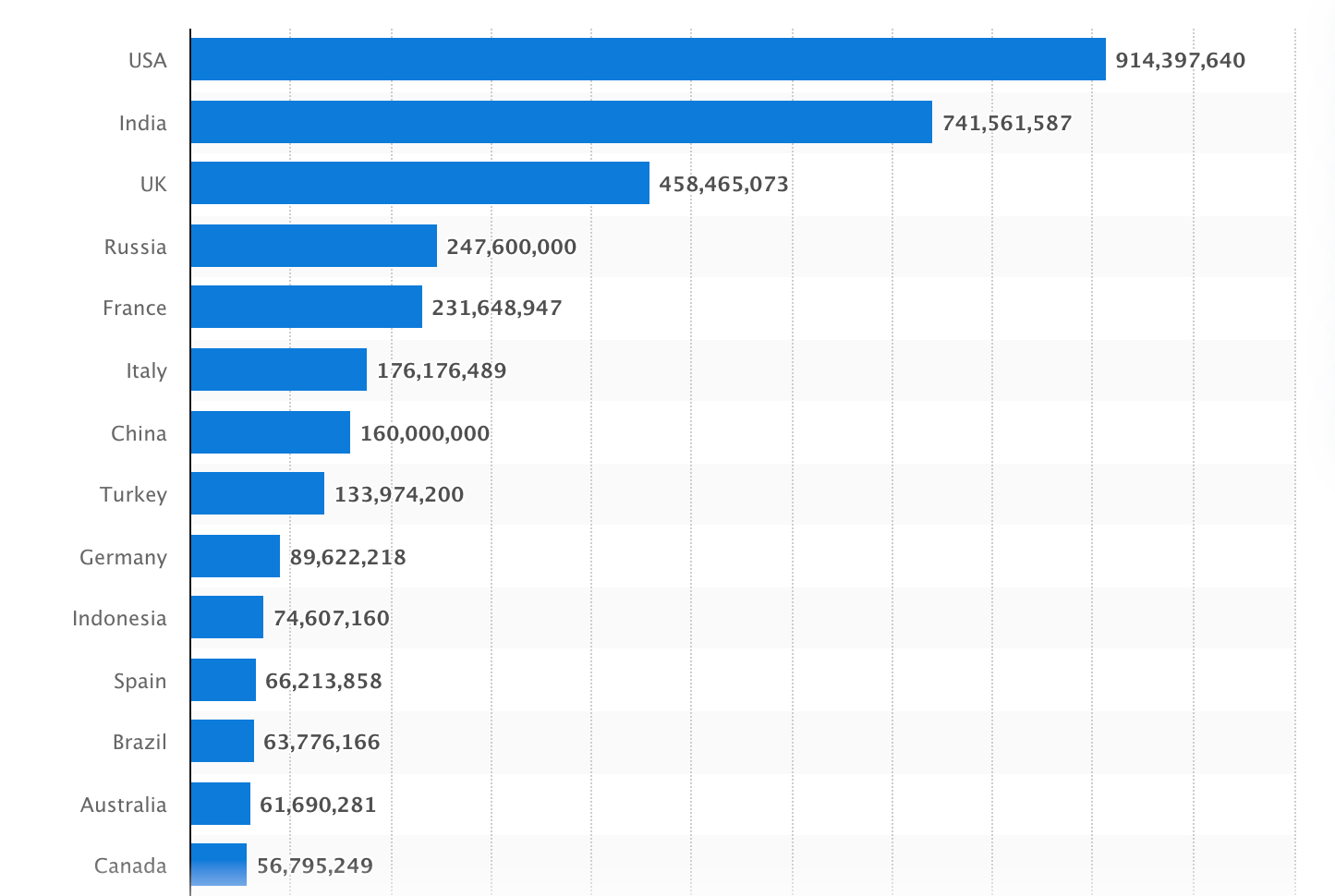
Covid testing puts incredible pressure on lab personnel, equipment, and software, and the risk of misplacing specimens or mixing up test results grows exponentially. The same goes for pharma research facilities working 24/7 on new drugs to fight the coronavirus and its consequences. But even if we take Covid out of the equation, 70% of medical decisions still rely on lab test results, according to the CDC.
Clinical and research laboratories urgently need software that can take the efficiency of data management to another level. Developing a lab management system can achieve just that.
So, how does a LIMS work its magic? Let's go behind the scenes and look inside the system.
Key features of LIMS software and the benefits they generate
When it comes to custom LIMS software, configurations depend on the specific needs of different medical businesses. However, to make a lab management system work, you must have the following typical components:
-
Sample tracking. One of the essential functionalities of a LIMS. This feature completely automates the process of tracking samples as they move through the lab and between departments. By assigning unique identifiers, the software creates a digital trail which leaves no room for human error.
-
Test workflow management. This module contains standardized routines for all of your tests. Additionally, it informs you about the types of tests, their results, validation status, and so on—another big step towards automation.
-
Centralized secure data storage. Following best practices in data storage is critically important for any LIMS. Personally identifiable information (PII) should be kept separate from test results, and security requirements must be fulfilled to prevent leaks. You'll also want quick and reliable access, with no downtimes that could disrupt the work of your lab.
-
Inventory & instrument control. Your lab should always be well-stocked with test supplies and chemicals to prevent delays. The software can trigger automatic alerts when restocking is due. Your LIMS can also monitor the equipment status and send prompt requests for maintenance.
-
Analytics & reporting module. This feature will help you reduce manual labor and improve your lab's efficiency. Export your data and make it available for internal departments or external clients who ordered the tests. Use advanced analytics and mine your data for insights on health issues, demographics, or your facility's expenses.
-
Regulatory compliance management. The healthcare industry is heavily regulated, which makes it too laborious to keep track every requirement. Avoid legal problems by adopting a lab management system that has built-in compliance tools — and update them regularly. We'll discuss compliance in more detail in one of the sections below.
Along with these features, you can request specific tools for implementation during LIMS software development. It could be explicit security controls to assign specific roles and permissions or additional data analytics modules. You could also ask your software partner to provide integration options for your EHR, PMS, or CDSS software.
By the way, that's another reason for your LIMS application to support data interoperability. If you want your lab data to move smoothly across departments or be used by external parties, storing it in a universal exchange format is a must.
As you can see, creating a LIMS generates a lot of benefits for a clinical or a research laboratory. With a LIMS tailored to your needs, you can:
-
Automate and standardize routine tasks
-
Streamline lab workflows
-
Eliminate human error
-
Leverage enhanced analytics
-
Improve security and compliance
-
Minimize lab delays and prevent downtime
-
Optimize equipment and supply-related expenses
-
Improve overall lab efficiency
-
Save costs and increase revenue
You're now familiar with the core features and upsides of a LIMS. Let’s move forward to potential downsides and how to avoid them.
Challenges of adopting a LIMS solution

Creating a LIMS system is a good idea for many healthcare organizations. But, to get a more balanced view, we'll tell you about the possible complications of LIMS software development.
Migrating data
Once you decide to make a LIMS part of your digital transformation, you’ll have to enter lots of data manually. And even if you're just launching your startup or switching from some siloed legacy software, data migration won't necessarily be lightning fast. But don't lose your spirit just yet.
In the past, software providers often had to devise custom data migration tools to suit their clients’ needs. These days, open-architecture software can easily take care of the problem. However, an expert should still oversee the migration process to ensure that no data is lost or corrupted.
Interoperability bottlenecks
More often than not, legacy laboratory management systems operate within data silos. Test results have to be entered manually into EMRs; data exists in several incompatible formats — the list goes on.
You'll definitely want your new LIMS to work with the latest interoperability standards in healthcare. To do that, you may have to reformat your data or even update the legacy software that you plan to integrate.
Training your staff to use the new system
People working in your lab are, most probably, highly trained professionals. However, they also have their own routines. Switching to a new LIMS application will take away a lot of manual tasks and change certain procedures. Your employees will need time and training to get the hang of the new system. Be prepared for a possible drop in performance during the transition period.
Ok, now you've seen both the peaks and the pitfalls. Let's walk further down the road and talk about the basics of LIMS development.
The issue of regulatory compliance

To use a laboratory information management system commercially, you need to ensure that it
complies with the relevant regulations. In addition to that, just like physical instruments and test methods, software must be validated. Validation here is the process of confirming that the software meets its intended requirements and follows quality procedures.
In the US, regulatory compliance for healthcare is governed by a number of organizations and laws. The chief regulator in the case of LIMS is Food and Drug Administration (FDA).
Here are some of the standards applicable to medical laboratories:
-
Health Insurance Portability and Accountability Act (HIPAA) — the act regulates all aspects of handling personal healthcare data for US-based businesses
-
Clinical Laboratory Improvement Amendments (CLIA) — if your activities include laboratory testing on human specimens
-
FDA's 21 CFR Part 11 — if your business involves manufacturing and/or selling drugs or medical devices
-
GDPR — the EU's equivalent of HIPAA, with even stricter provisions
-
ISO 17025 — quality standard for all laboratories that do testing and calibration
Having a validated, compliant LSIM is imperative for your medical business to avoid penalties and fines. Validation is usually a joint effort between your company and the software partner. Your project team should assist you in meeting your custom validation needs.
Building a laboratory information management system that's right for you

We compiled this short guide based on our 6+ years of experience of building software for various clients in healthtech. Here’s what the process of creating a LIMS system looks like.
Assess your needs and pain points
This sounds like an obvious thing to do, but the success of the whole endeavor depends on this step.
Start by evaluating the current and potential strengths and weaknesses of your business. What are the bottlenecks in the lab’s operation? Can the adoption of a LIMS effectively solve your issues? For instance, you might find out that your equipment is too outdated to keep up with the new software. Or that part of your problem is a lack of qualified or motivated personnel.
List down product requirements
To get a crisper view of your future software, you'll also need to formulate product requirements. They should cover the functional side of the LIMS — its features and the way they work — as well as expectations in terms of security, stability, responsiveness, and so on.
Your product requirements document (PRD) will serve as a blueprint for the development team.
Build the software
At this point, your software partner should have enough information to start creating your LIMS.
The team will get on with planning, designing, coding, and testing. It’s crucial to be in constant contact at this stage to track progress.
Don't hesitate to ask for changes or additions — they will be much harder to implement later down the road. Quality custom software takes time and effort to develop, but the end product must be tuned to your needs.
Begin implementing your new LIMS
Once your software is released and deployed, you can start onboarding. If the product is a solid, well-designed piece of software, your staff should be able to learn the ropes quickly. Your software partner should provide the necessary assistance, smoothing out any issues during the training.
You can now enjoy your new lab information management system and reap its benefits. Congratulations on the fine work you've done together with your software partner.
Oh, by the way. Why not talk about how to pick the right team?
How to look for the right development team?

Sooner or later, you'll have to find a team of professionals who can convert your ideas into a fully functional, reliable product.
You can get the ball rolling by browsing websites like G2 and Clutch for up-to-date and unbiased information.
Keep in mind your specific needs and compare them with the providers' descriptions. Can this company cover your requirements, and do they have the healthtech expertise? Look through reviews and client testimonials, so it's easier to make an informed decision.
Once you narrow the choice down, get a better feel of the candidates by visiting their websites. You'll find extra info on the number and types of projects they've completed, along with a detailed description of their services and tech stack.
Got a winner? On to the next step.
Get in touch, set up a meeting online, and talk to the team. Give the gist of your project and exchange ideas. If your first encounter goes well, you can move on to discuss the details.
Communicate your goals and expectations, describe the specifics of your business model, ask questions, and be ready to ask more. You'll need to determine the project scope, discuss the features you need, set a timeline, and plan a budget.
If you successfully arrived at a common vision and agreed on the project’s technical and financial aspects — you've just found yourself a perfect software partner.
Choose a great team of experts to develop your LIMS
Adopting a LIMS is a great way to make your lab more efficient while dealing with additional loads and new types of tests. It's also a major step towards better automation and harnessing the power of healthcare data.
It can be a tough call to find developers experienced in lab management software development. Should you need any help, the Demigos team is ready to offer our services. We can walk you through the choice of features, help you migrate data, ensure compliance, and build LIMS software that will fit your needs.
Let's get in touch and start creating together.






